Sharpsmarts Leading-Edge Container Washing

Bioburden reduction in a robotic container washing process is a REVOLUTIONARY achievement in the medical waste industry.
When it comes to safety and the infection protection of staff and patients, there are simply no shortcuts. Sharpsmart’s peerless reputation for “innovation that impacts” does not stop at our world-leading sharps and medical waste containers, in fact it extends to every process, system and technology that supports Sharpsmart's solutions both within the four walls of a healthcare facility and at our own clean processing facilities.
Every Sharpsmart container is robotically washed and disinfected
Every time a Sharpsmart reusable sharps or pharmaceutical waste container is returned to a Sharpsmart facility, it is processed on one of our Washsmart robotic washing conveyers, a state of the art piece of machinery patented by Sharpsmart and proven to achieve unprecedented results in bacterial load reduction. The 6-step decanting and washing process is automated to eliminate human risk exposure, and utilizes a mix of environmentally friendly detergents to ensure each sharps or medical waste container is rendered free from chemical residue. Each Washsmart machine is made with over 15,000 parts and uses a tested combination of water pressure, high temperatures and biodegradable detergent to achieve high level disinfection. Through its rigorous decontamination cycle, the risk for infection and HAI’s is greatly reduced.
Sharpsmart's Washsmart results are unprecedented in the healthcare industry
Sharpsmart's Washsmart system has been extensively researched, tested and independently verified as achieving a level of sterilization 4x higher than required by the CDC in the United States. During the decontamination process, blood and human-source soiling are completely removed and the inside of the container is made safe for handling, the Sharpsmart Washsmart has been tested against the Ninhydrin test (a forensic level test to detect traces of protein or blood) and has also recently achieved the first ever results for removal of high-density nontoxigenic spores of C. Difficile. The full article can be read in the American Journal of Infection Control here.
What are the regulations surrounding reusable containers?
Reusable sharps or medical waste containers are ultimately “waste containers”. They do not generally come in contact with patients and have not been cited in disease transmission to patients or staff. For example, the US Centers for Disease Control regard such surfaces as low-touch environmental surfaces and as such require “thorough cleaning”.1
Sharpsmart's Washsmart process achieves High-level Disinfection - a decontamination level used for semi-critical patient instruments,2 and considerably above relevant guideline requirements in the United States, Canada, and Australia.2,3,4 The CDC state, “High-level disinfection traditionally is defined as complete elimination of all microorganisms in or on an instrument, except for a small numbers of bacterial spores. The FDA definition of high-level disinfection is a sterilant used for a shorter contact time to achieve a 6-log10 kill of an appropriate Mycobacterium species.”2
How Sharpsmart's Washsmart achieves its significant bioburden reduction results
The time, temperature and water volumes necessary to achieve this level of cleaning are closely monitored. If any parameter falls outside Sharpsmart's set criteria, the Washsmart machine immediately shuts itself down so it can be repaired. The steps of the process are as follows:
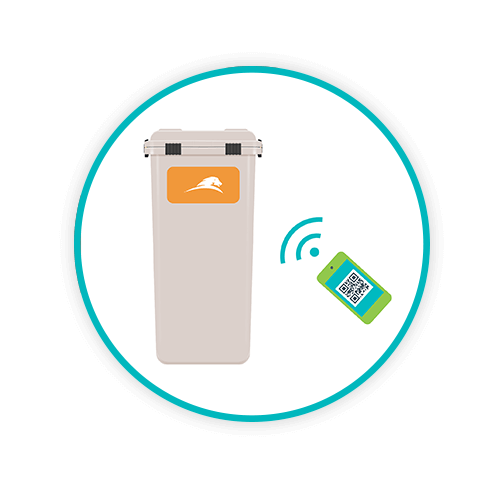
WEIGH AND SCAN
Each collector is loaded onto a robotic conveyor belt; contents are weighed and the collector barcode is scanned to enable complete traceability of its origins.
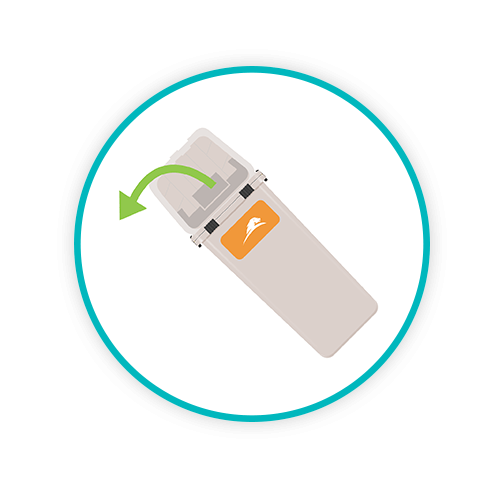
DECANT AND EMPTY
In a seamless automated action, the robotic arms of the Sharpsmart's Washsmart tip the collector upside down to expel contents into a large holding bin situated beneath the machine.
HIGH PRESSURE FLUSH
Strong jet streams of cold water are injected into the collector to flush out any stubborn residue, at a high pressure force, this flushing process removes dry blood retained debris or residual fluids.
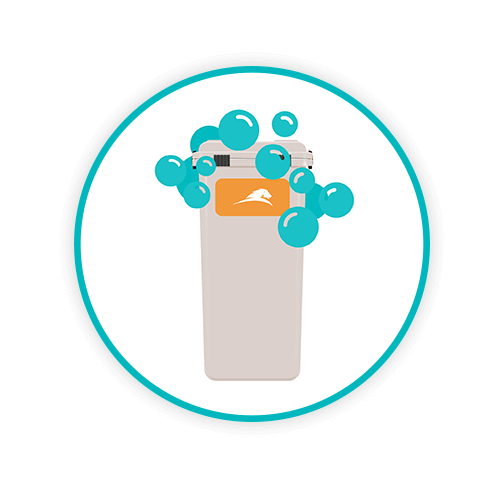
WASH
The collector undergoes a hot water hypochlorite wash up to a maximum temperature of 131°F for 45 seconds with a concentration enabling full coverage of all surfaces.

HOT RINSE
The collector enters into a secure chamber where it undergoes a hot scald rinse at 185°F for 14 seconds.
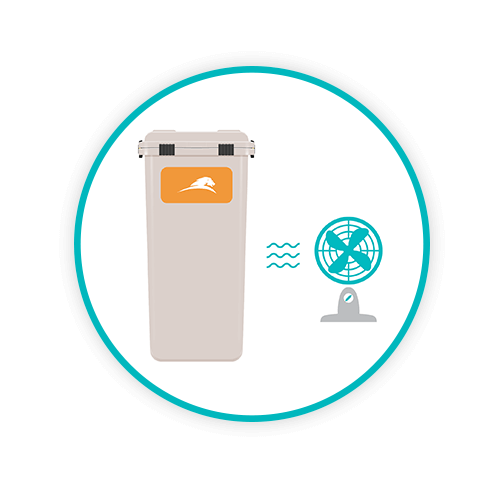
DRYING
The final stage of the Washsmart process is to remove surface water, thereby eliminating the possibility of subsequent microbial growth; this process is undertaken via a blow-dry mechanism.
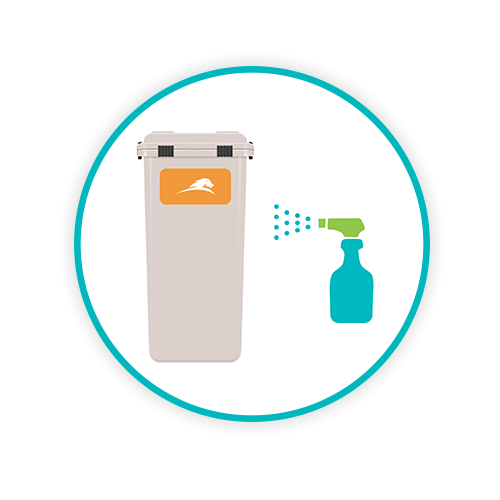
SMARTGUARD
Sharpsmart has a unique coating formulation which is applied at every wash to enable Sharpsmart collectors to easily shed sticky adhesives, blood, body fluids, dyes and any debris or organic matter.
The result of the Washsmart process is a 6Log reduction in bio burden – some 1000 times greater than that required for thorough cleaning. At the end of the process each Sharpsmart container is QA inspected for function, scuffs, cuts and smears. The fully automated Sharpsmart collector sanitization process is engineered deliberately for microbial overkill whether the container holds cytotoxic waste, expired pharmaceuticals, sharps or clinical waste. The three actions in the process: physical, detergent, and heat, render the Sharpsmart sharps container and Clinismart containers free of pre-wash contamination and hygienic for reuse while having negligible impact on the environment. We’re pretty proud of our Washsmart innovation! It’s one of the many ways in which Sharpsmart guarantees safety within the waste management process whilst also ensuring we’re upholding the highest measure of sustainability. To learn more about our washing and treatment processes, visit our Operations page here.
1 Centers for Disease Control and Prevention Guidelines for Environmental Infection Control in Health-Care Facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC), 2003. U.S. Dept HHS, (CDC) Atlanta, GA. https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines.pdf.
2 Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. A Rational Approach to Disinfection and Sterilization. Centers for Disease Control and Prevention, Atlanta GA, USA. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/rational-approach.html
3 British Columbia Best Practices for Environmental Cleaning for Prevention and Control of infections in All Healthcare Settings and Programs. Provincial Infection Control Network of British Columbia (PICNet). Vancouver, BC, Canada. Sept 2016.
4 NHMRC (2010) Australian Guidelines for the Prevention and Control of Infection in Healthcare. Commonwealth of Australia. National Health and Medical Research Council and Australian Commission for Safety and Quality in Health Care. https://www.nhmrc.gov.au/about-us/publications/australian-guidelines-prevention-and-control-infection-healthcare-2010
